Ultrasound of the Month: Central Retinal Artery Occlusion
The Case
70-year-old male with a history of hypertension, hyperlipidemia, and age-related cataracts presented for acute vision loss in his left eye that started while sitting in a car, approximately eight hours prior to arrival. When closing this eye, he reported painless “splotchy” vision with some areas where light was able to shine through. He spoke with his ophthalmologist who sent him to the ED for an emergent ophthalmologic evaluation. He denied numbness, weakness, tingling, headaches, temporal pain, or changes in the vision in his right eye. He reported no recent illnesses, trauma, or head strike.
Ophthalmology was consulted, and while awaiting their evaluation, an ocular ultrasound was performed.
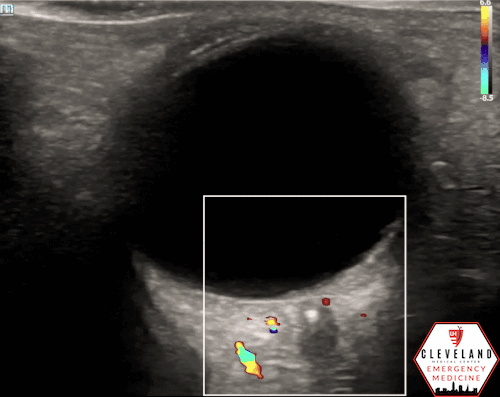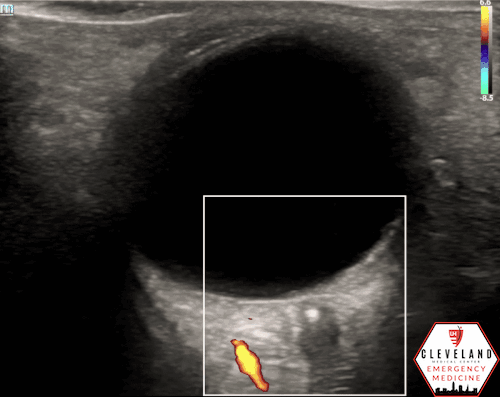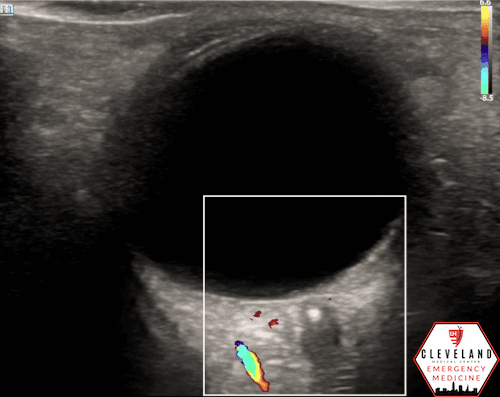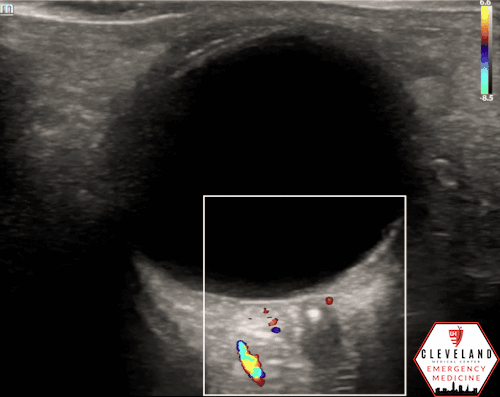
POCUS Findings: Ocular ultrasound revealed a small hyperechoic focus over the distal optic nerve in the retrobulbar space, raising concern for central retinal artery occlusion (CRAO) in the setting of painless monocular vision loss. Color doppler demonstrated diminished arterial flow in his region, further supporting the diagnosis.
Case Conclusion: Ophthalmology performed a dilated fundoscopic exam, which revealed a cherry-red spot over the left macula, confirming the diagnosis of CRAO. The patient was admitted for further management and workup.
Central Retinal Artery Occlusion
Central retinal artery occlusion (CRAO) is a relatively rare ocular emergency, with an incidence of approximately 1.0 per 100,000 person-years in the United States. It primarily affects male patients, with a mean age of 60 to 65 years (1). CRAO presents with sudden, painless monocular vision loss. Often referred to as the ocular equivalent of a cerebral stroke, its visual prognosis is usually poor — approximately 61% of patients retain vision no better than counting fingers (2).
In 2013, the American Heart Association and American Stroke Association updated their stroke classifications to include CRAO and branch retinal artery occlusion (BRAO). Like cerebral ischemic strokes, retinal artery occlusions result from thromboembolic events that cause vascular occlusion and lead to end-organ ischemia. In CRAO, the occlusion occurs within the central retinal artery, a branch of the ophthalmic artery that supplies the retina (see Figure 1) (2). The most common site of occlusion is the narrowest portion of the artery, where it passes through the dural sheath of the optic nerve (3). Dilated fundoscopic examination is the gold standard for diagnosis, typically revealing a pale retina with a characteristic “cherry-red spot.” However, this exam is often impractical in the emergency department, where ophthalmology consultation may not be readily available. Point-of-care ultrasound (POCUS) may be a valuable adjunct to support a timely diagnosis. CRAO that is not reversed within four hours results in severe retinal ischemia, and recovery of vision is rarely possible after that window (4).
Figure 1. A). Diagram of the course of the central retinal artery, a branch of the ophthalmic artery, showing the location of a CRAO (green X). B). Fundus photograph showing cherry-red macula (M). Image credit Chen et al. 2024 (2).
Ocular Ultrasound
While ocular ultrasound is not routinely part of the standard diagnostic workup for CRAO specifically, it is a valuable and effective tool for evaluating other causes of painless vision loss, such as retinal detachment, vitreous detachment, and vitreous hemorrhage (5). The exam should be avoided if globe rupture is suspected, as this is one of the few absolute contraindications to ocular ultrasound.
Image Acquisition
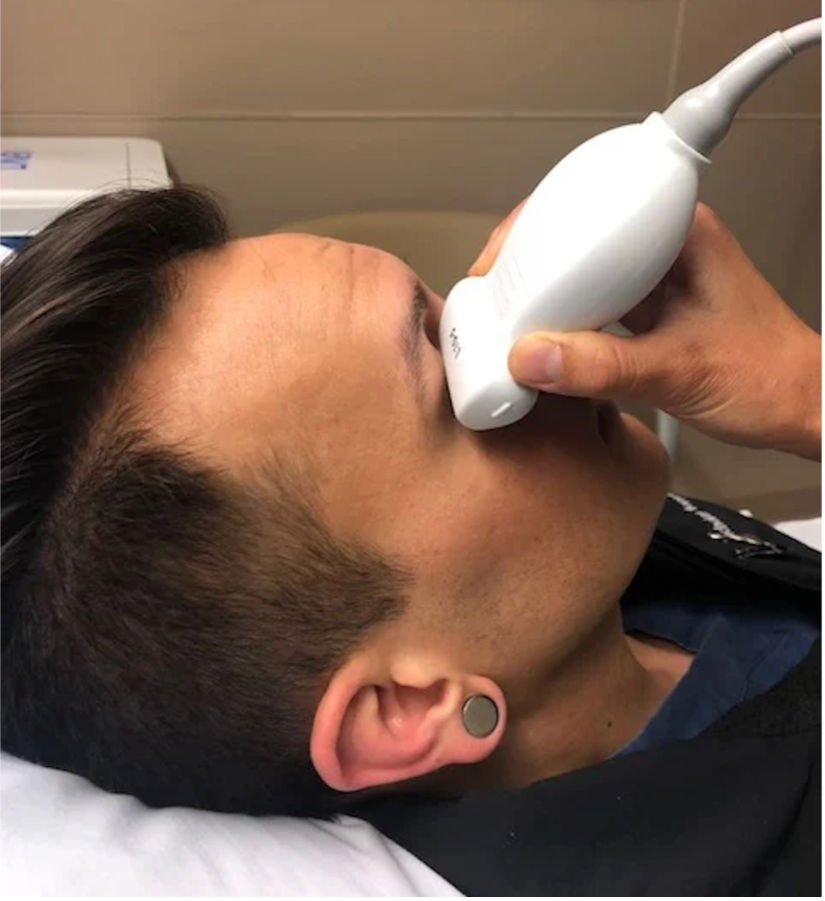
Begin by placing a tegaderm over the closed eyelid to prevent ultrasound gel from getting into the eye. When performing ocular ultrasound, it is essential to minimize pressure on the globe. To achieve this, use an abundance of gel to allow for minimal to no applied pressure. To help stabilize your hand and avoid applying too much force, rest your fifth digit on the patient’s nose or temple while scanning.
Use a high-frequency linear probe to scan in both transverse (demonstrated in Figure 2) and sagittal planes. Adjust the depth so the globe is centered in the image and the optic nerve sheath is visible posterior to the globe. This appears as a hypoechoic structure extending from the posterior aspect of the vitreous body (see Figure 3). Increasing the gain may help identify subtle findings within the vitreous body.
Be sure to include a dynamic (kinetic) exam by asking the patient to move their eyes during scanning. In the transverse plane, ask them to look left and right; in the sagittal plane, ask them to look up and down. This technique improves visualization and helps differentiate mobile versus fixed pathology (6).
Figure 2. Probe placement. Image credit: ACEP Sonoguide (6).
Figure 3. Sonographic anatomy of the eye
“Retrobulbar Spot Sign”
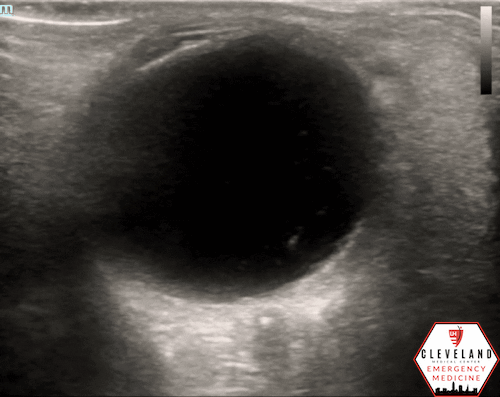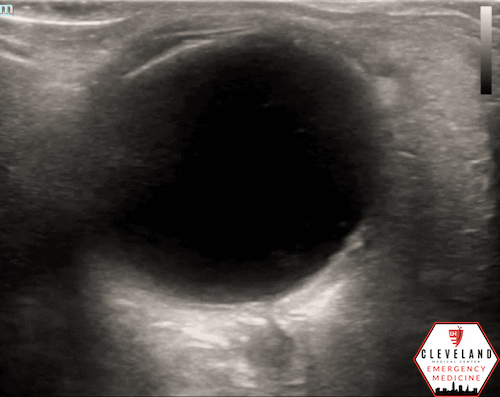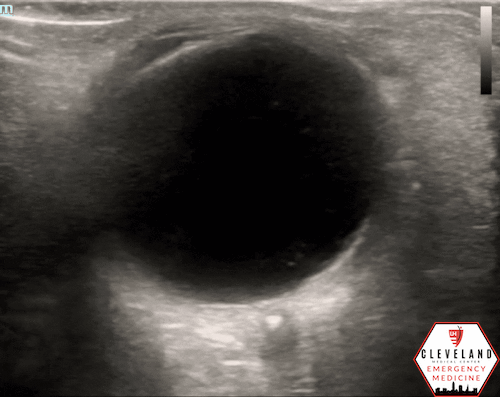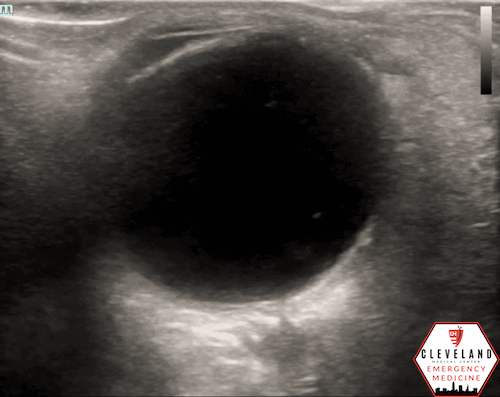
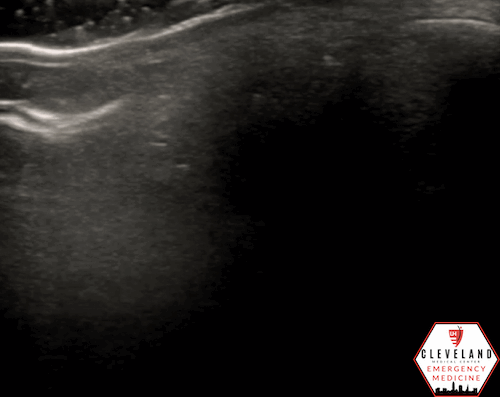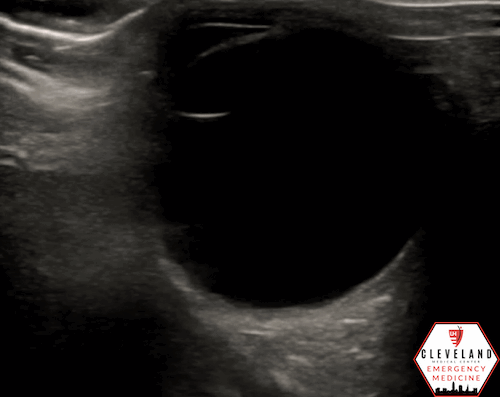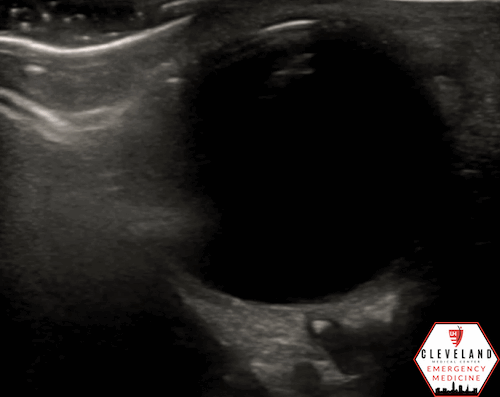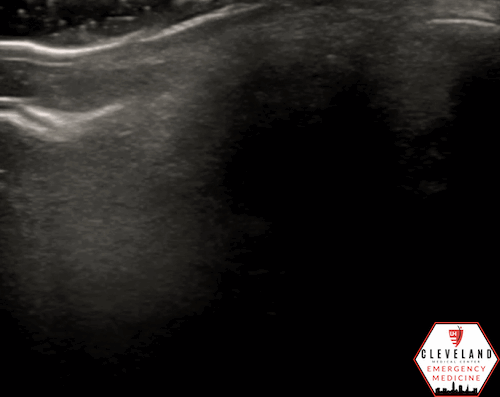
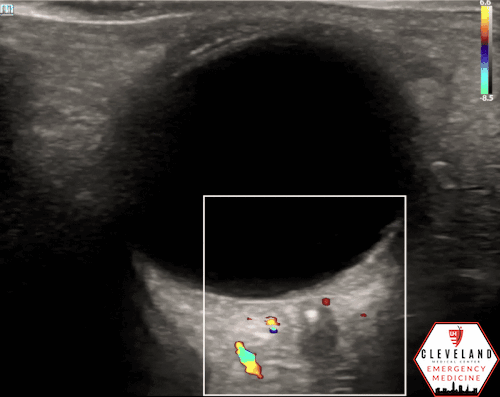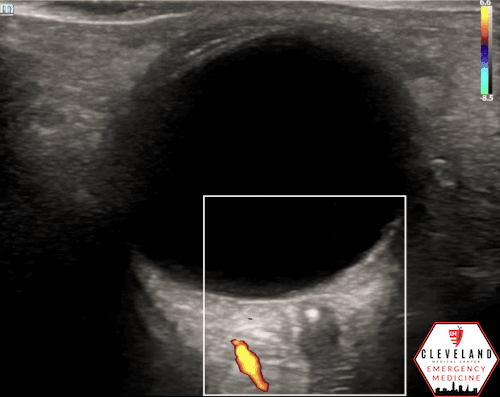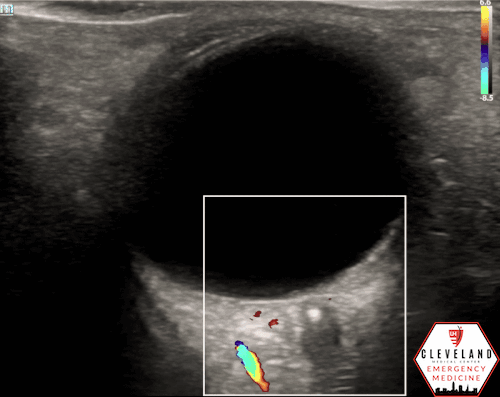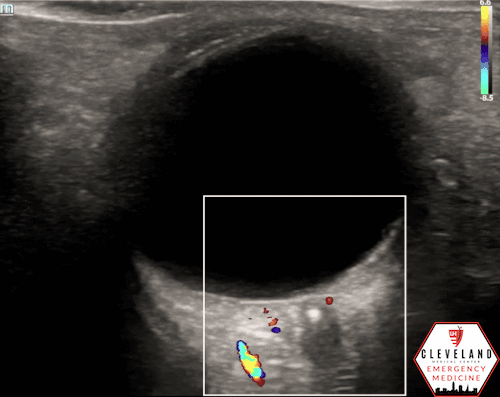
A hyperechoic focus over the distal optic nerve — referred to as the retrobulbar spot sign — has been observed on ocular ultrasound in cases of CRAO and may help differentiate it from other causes of painless monocular vision loss (7,8). Illustrated in Figures 4-6, this finding is considered highly specific for embolic CRAO and, when present, is associated with a significantly lower likelihood of temporal arteritis (9). However, its true prevalence remains unclear, and most studies evaluating its diagnostic accuracy have been small. In one study, the retrobulbar spot sign demonstrated a sensitivity of 83% and specificity of 100% for CRAO (9).
If a hyperechoic spot is identified, consider using color Doppler to assess for diminished or absent arterial flow distal to the focus (see Figure 6). Keep in mind that Doppler signal is angle-dependent, and poor probe positioning may limit its reliability.
Given the limited evidence, a dilated fundoscopic examination remains the gold standard for diagnosing CRAO. However, POCUS can still serve as a valuable adjunct in the emergency setting — aiding in earlier diagnosis, expediting stroke workup, and facilitating timely management, especially when ophthalmologic evaluation is delayed or unavailable.
Figure 4. Sagittal and transverse views of a retrobulbar hyperechoic spot (8).
Figure 5. Retrobulbar hyperechoic spot sign from our patient’s case
Figure 6. Color doppler shows lack of arterial flow over the optic nerve
Figure 7. Optic nerve drusen, a potential mimicker of the CRAO spot sign
Potential Mimicker: Optic Nerve Drusen
Optic nerve drusen can mimic the retrobulbar spot sign seen in CRAO, as both appear as hyperechoic foci overlying the distal optic nerve. However, drusen are typically larger, are often more irregular in shape, and are located at the very distal portion of the optic nerve, near the retinal surface — sometimes even protruding into the vitreous (see Figure 7). In contrast, the hyperechoic foci associated with CRAO are usually smaller, more discrete, and located slightly deeper in the retrobulbar space (6, 10).
More Common Pathology
While ocular ultrasound can support the evaluation of CRAO, it is most commonly used to assess for retinal detachment and vitreous pathology, such as vitreous hemorrhage or detachment — which are more frequent causes of acute painless monocular vision loss. For a deeper dive into their sonographic features, check out any of our related posts, such as Retinal Detachment, Vitreous Hemorrhage.
Take-Home Points
CRAO presents as painless monocular vision loss and is a true ocular emergency.
Dilated fundoscopic exam remains the gold standard, but POCUS can aid early diagnosis when an ophthalmology evaluation is delayed or unavailable.
A retrobulbar spot sign is not always present—but when it is, it strongly supports embolic CRAO in the appropriate clinical context.
Scan both eyes for comparison, and consider using color Doppler to strengthen your findings.
For any ocular ultrasound exam, always include a dynamic (kinetic) exam to help identify subtle or mobile pathology within the vitreous body.
Optic nerve drusen may mimic the spot sign but are typically larger and located more distally near the retinal surface.
AUTHORED BY: HANNAH HENDRIX, MD, PGY2
FACULTY CO-AUTHOR/EDITOR: LAUREN MCCAFFERTY, MD
References
Sim S, Ting DSW. Diagnosis and management of central retinal artery occlusion. American Academy of Ophthalmology. August 1, 2017. Available from: https://www.aao.org/eyenet/article/diagnosis-and-management-of-crao.
Chen C, Singh, G, Madike R, and Cugati S. Central retinal artery occlusion: A stroke of the eye. Eye. 2024. Available from https://www.nature.com/articles/s41433-024-03029-w.
Kang D, Jung, K, Yang W, et al. Presence of embolic source and outcome in central retinal artery occlusion. Neurology. 2023;101(13): e1364-e1369.
Hayreh SS. Acute retinal arterial occlusive disorders. Prog Retin Eye Res. 2011 Sep;30(5):359-94.
Lahham S, Shniter I, Thompson M, et al. Point-of-care ultrasonography in the diagnosis of retinal detachment, vitreous hemorrhage, and vitreous detachment in the emergency department. JAMA Netw Open. 2019;2(4):e192162.
Situ-LaCasse E, Adhikari SR. Ocular Emergencies. ACEP Sonoguide. August 18, 2020. Retrieved from https://www.acep.org/sonoguide/advanced/ocular-emergencies.
Usheva E, Williams D, Musgrave H, Zhou S. Sonographic retrobulbar spot sign in diagnosis of central retinal artery occlusion: A case report. JETem. 2023. 8(4):V5-8.
Caja KR, Griffith KM, Roth KR, et al. Detection of central retinal artery occlusion by point-of-care ultrasound in the emergency department: A case series. Cureus. 2021;13(7):e16142.
Ertl M, Altmann M, Torka E, et al. The retrobulbar "spot sign" as a discriminator between vasculitic and thrombo-embolic affections of the retinal blood supply. Ultraschall Med. 2012 Dec;33(7):E263-E267.
Core Ultrasound. CRAO. https://coreultrasound.com/crao/