Ultrasound-Guided Pericardiocentesis: A Review
Introduction
Pericardiocentesis is a procedure performed to remove excess fluid from the pericardial sac surrounding the heart. It is most often indicated when cardiac tamponade develops, an emergent and potentially fatal condition. In this setting, pericardial fluid exerts extrinsic pressure on the heart, impairing venous return. On echocardiography, tamponade manifests as right atrial systolic collapse followed by right ventricular diastolic collapse, ultimately leading to reduced cardiac output [1]. Clinically, cardiac tamponade is classically suggested by Beck's triad: hypotension, jugular venous distention, and distant heart sounds.
Indications and contraindications
Indications
The primary indication for pericardiocentesis is a pericardial effusion causing tamponade. Emergent pericardiocentesis is required when tamponade causes hemodynamic instability or impending arrest, whereas non-emergent pericardiocentesis may be performed for symptomatic effusions, large effusions at risk of tamponade, or for diagnostic purposes [2].
Absolute contraindications
While pericardiocentesis can be a life-saving procedure, certain conditions, such as ventricular free wall rupture and aortic dissection, are absolute contraindications. In these cases, pericardiocentesis is generally avoided but may still be considered as a temporizing measure until definitive surgical interventions are available. Relative contraindications include anticoagulation therapy, uncorrected coagulopathy, and thrombocytopenia (platelets <50,000), as these increase bleeding risk. In such cases, the risks and benefits should be carefully weighed, but tamponade with instability remains an indication to proceed [2,3].
Supplies
18-20 gauge pericardiocentesis (or spinal) needle
30 cc syringe (or larger)
Ultrasound machine
Chlorhexidine swab*
Sterile gloves*
Sterile probe cover*
Sterile drape*
*in ideal circumstances, depending on the urgency of the procedure
Technique
Pericardiocentesis may be performed from the subxiphoid, parasternal, or apical approaches. The optimal window will depend on the patient’s body habitus and anatomy. The optimal needle entry site is where ultrasound demonstrates the largest and most superficial pocket without close proximity to vital structures [3].
General Steps
Identify the effusion and select the safest window with phased-array probe.
Optimize your image: decrease the depth so the effusion and proximal cardiac structures are centered on the screen — this improves resolution.
Consider switching to a linear probe after localization for better needle visualization.
Prep the skin with chlorhexidine; apply sterile drape and probe cover, if time permits.
Introduce a 20-gauge (or larger) needle under real-time ultrasound guidance at ~45° using an in-plane approach, carefully advancing toward the pericardial space.
Aspirate continuously until pericardial fluid is obtained [4,5].
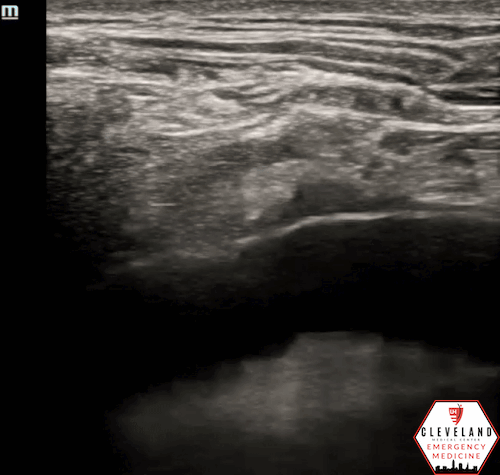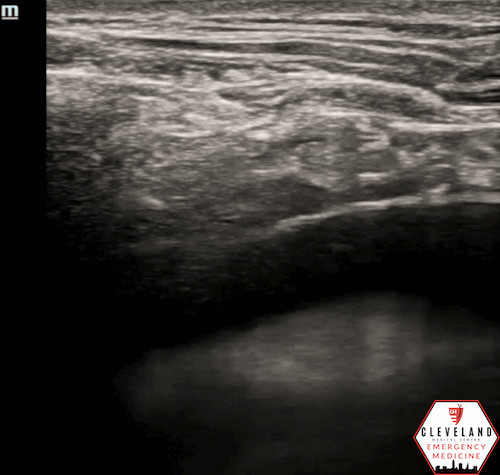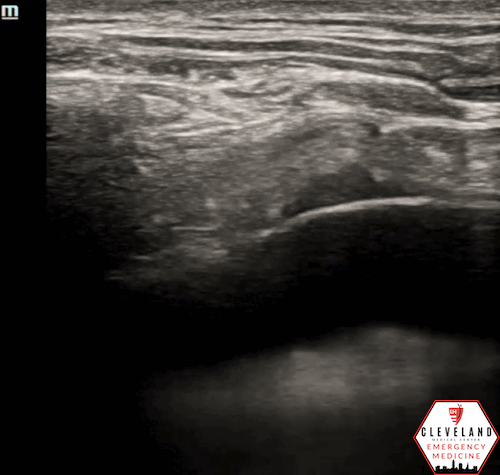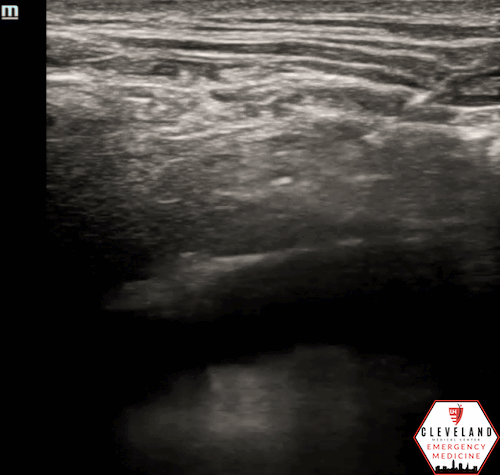
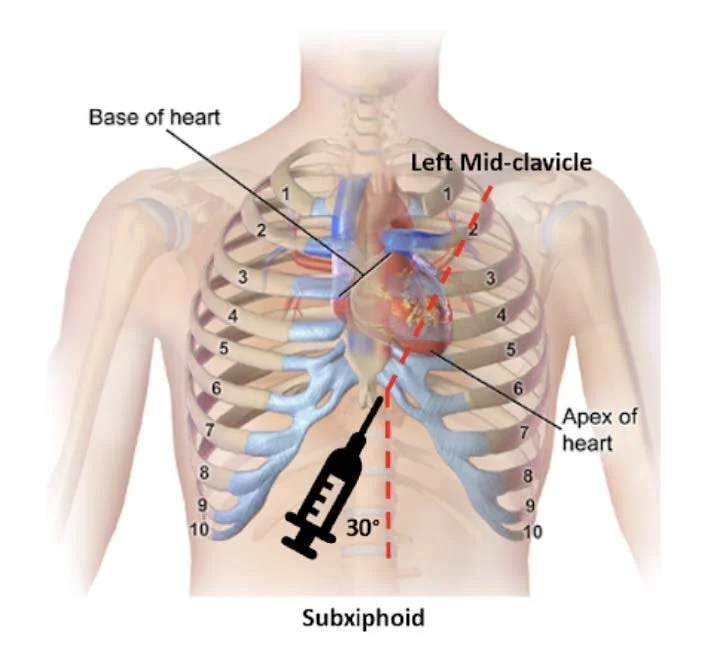
Figure 1. Subxiphoid approach - Source: NUEM Blog — Pericardiocentesis (nuemblog.com)
*The following cardiac views describe probe orientation based on a cardiac preset, with the indicator on the right side of the screen.
Subxiphoid Approach
Probe Placement: Transverse on the left costal margin, adjacent to the xiphoid.
Probe Orientation: Indicator directed toward the patient’s left shoulder.
Anatomy: Liver borders the right ventricle.
Needle: Insert at 45° relative to the skin, directed toward the left scapula.
Pros/Cons: Traditional landmark-based site. With ultrasound, visualization may be limited due to a deeper trajectory, and there is greater risk of liver or peritoneal injury compared with parasternal or apical approaches [4-5].
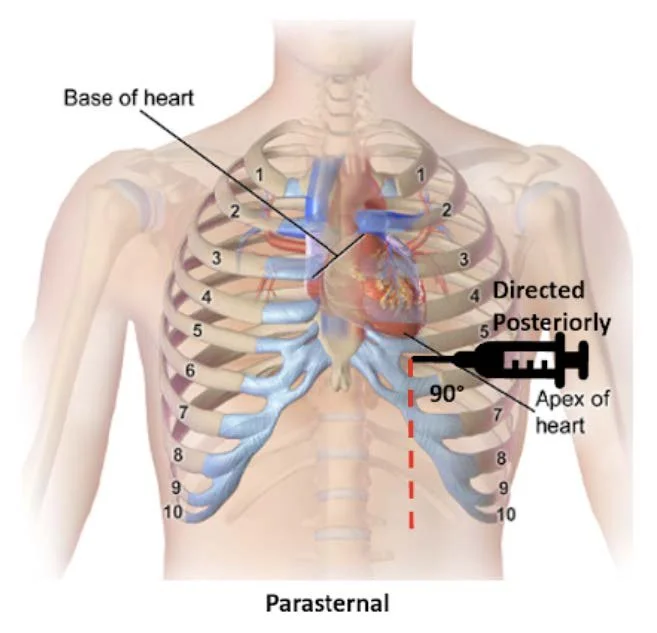
Figure 2. Parasternal approach - Source: NUEM Blog — Pericardiocentesis (nuemblog.com)
Parasternal Approach
Probe Placement: Left parasternal region, usually 4th intercostal space.
Probe Orientation: Indicator toward the patient's left shoulder.
Needle: Insert in-plane at ~45°, directed toward the effusion.
Pros/Cons: Often preferred due to closer proximity of effusion to the chest wall and distance from lung/liver. The needle trajectory aligns well with cardiac structures, improving needle visualization [4-5].
Figure 3. Apical approach - Figure 1. Subxiphoid approach - Source: NUEM Blog — Pericardiocentesis (nuemblog.com)
Apical
Probe Placement: 4th-5th intercostal space, under the left breast or inframammary fold, near mid-axillary line.
Probe Orientation: Indicator toward left axilla.
Needle: Insert in-plane at ~45°, advancing toward the pericardial space.
Pros/Cons: Similar to parasternal approach in advantages; the short distance from chest wall allows improved visualization and safety [4,5].
Complications
The overall complication rate for ultrasound-guided pericardiocentesis is low, with major complications in 0.3 to 3.9% and minor complications in 0.4 to 20% of cases [2,6]. Major complications include injury to cardiac chambers, coronary artery or intercostal vessel lacerations, pneumothorax, injury to abdominal viscera, ventricular arrhythmias, pericardial decompression syndrome, pneumopericardium, and death. Minor complications include dry taps, transient vasovagal hypotension, supraventricular arrhythmias, and pneumothorax not requiring a chest tube [7-8].
Take Home Points
Emergent pericardiocentesis is indicated for tamponade with hemodynamic instability or arrest.
Absolute contraindications include free wall rupture or aortic dissection, though drainage may be considered as a temporizing measure until surgery is available.
Ultrasound guidance improves safety; parasternal and apical approaches often provide better visualization, but the best window is the one that shows the effusion most clearly.
Major complications are uncommon but can be life-threatening.
AUTHORED BY: THOMAS EVANS, MD,
FACULTY CO-AUTHOR/EDITOR: LAUREN MCCAFFERTY, MD
References
Alerhand S, Adrian RJ, Long B, Avila J. Pericardial tamponade: A comprehensive emergency medicine and echocardiography review. Am J Emerg Med. 2022; 58:159-174.
De Carlini CC, Maggiolini S. Pericardiocentesis in cardiac tamponade: indications and practical aspects. CardioPractice. 2017; 15(19).
Loukas M, Walters A, Boon JM, Welch TP, Meiring JH, Abrahams PH. Pericardiocentesis: a clinical anatomy review. Clin Anat. 2012;25(7):872-81.
Mirza M, Bryczkowski C. Pericardiocentesis. Sonoguide. Published November 17, 2021. Accessed June 2024. https://www.acep.org/sonoguide/procedures/pericardiocentesis.
Swaminathan A. Core EM: Ultrasound-guided pericardiocentesis. emDOCs.net. Published September 9, 2015. Accessed June 2024. https://www.emdocs.net/core-em-ultrasound-guided-pericardiocentesis/
Tsang TS, Enriquez-Sarano M, Freeman WK, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002;77(5):429-36.
Maggiolini S, Gentile G, Farina A, et al. Safety, Efficacy and Complications of Pericardiocentesis by Real-Time Echo-Monitored Procedure. Am J Cardiol. 2016; 117 (8): 1369-74.
Akyuz S, Zengin A, Arugaslan E, et al. Echo-guided pericardiocentesis in patients with clinically significant pericardial effusion. Outcomes over a 10-year period. Herz. 2015; 40(2): 153-159.