Intern Ultrasound of the Month: AAA
The Case
80-year-old female with a past medical history significant for hypertension, hyperlipidemia, coronary artery disease status post coronary artery bypass graft, peripheral arterial disease, diverticulitis, PEG tube placement for chronic dysphagia presented to the emergency department for abdominal pain and confusion. The patient’s family reported that she had been complaining of nausea and abdominal pain for the past day. History from the patient was limited by her confusion but no other known symptoms. Prior CT angiography studies showed stable AAA under 5cm, which was being monitored by her physician.
Her physical exam was remarkable for abdominal tenderness in the right and left lower quadrants and suprapubic region. There was no guarding or rebound tenderness. No obvious neurovascular deficits noted.
Point-of-care ultrasound of aorta was performed to evaluate for change in AAA given her presenting symptoms.
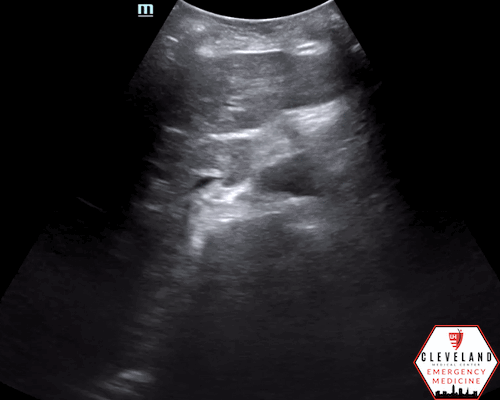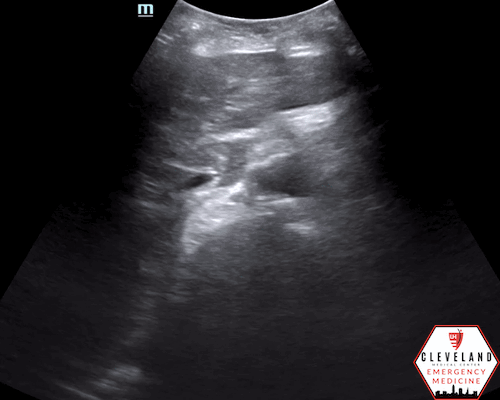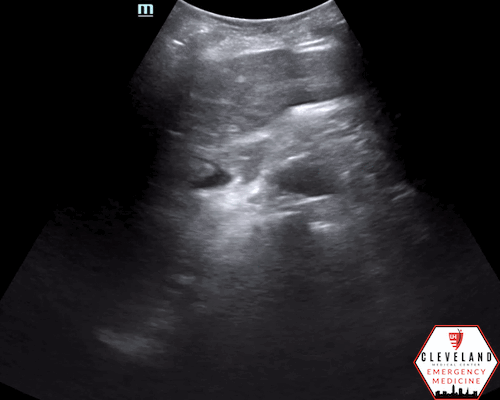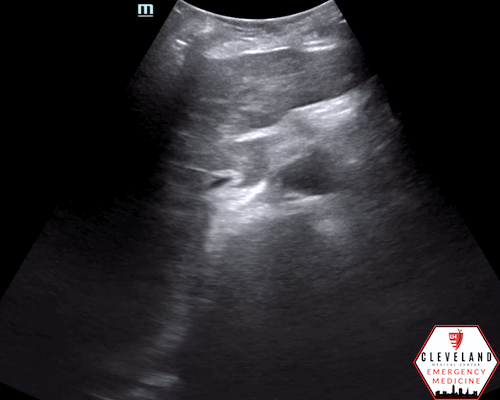
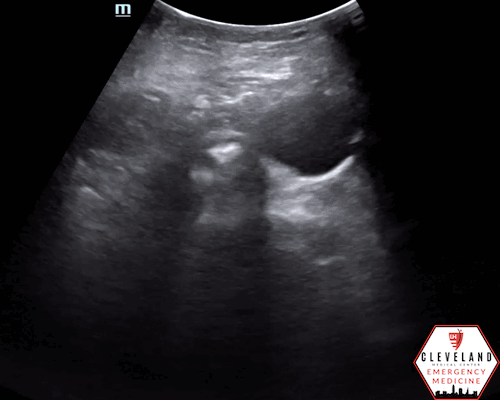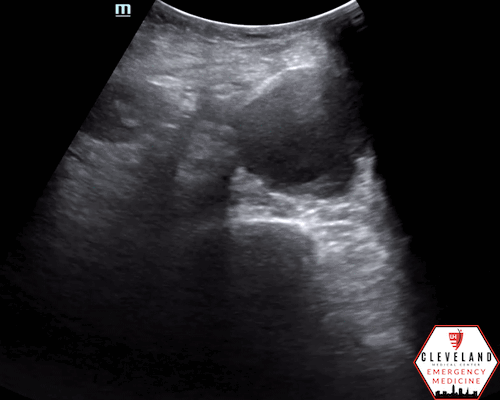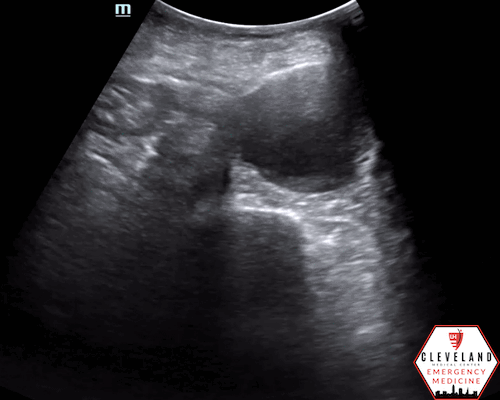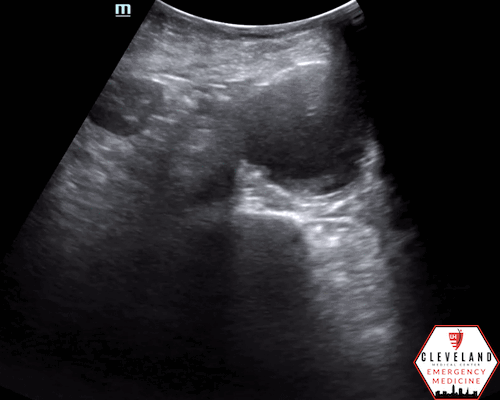
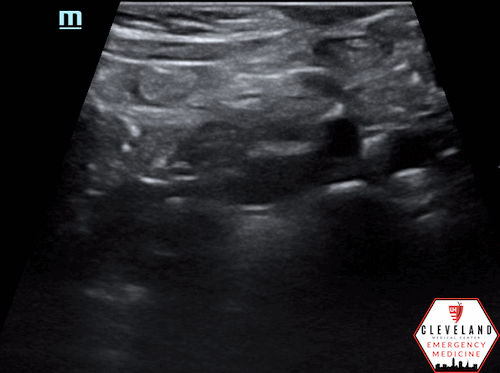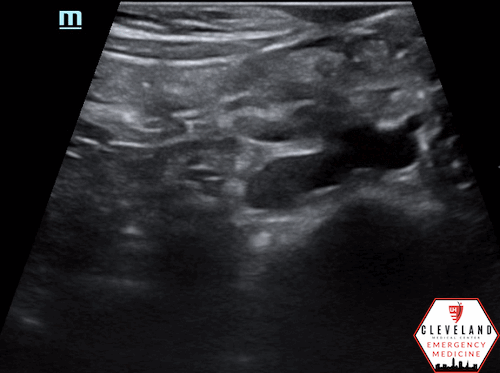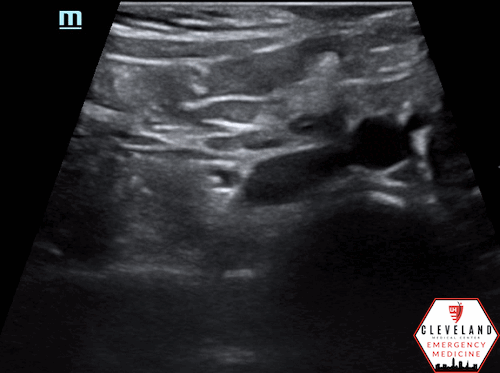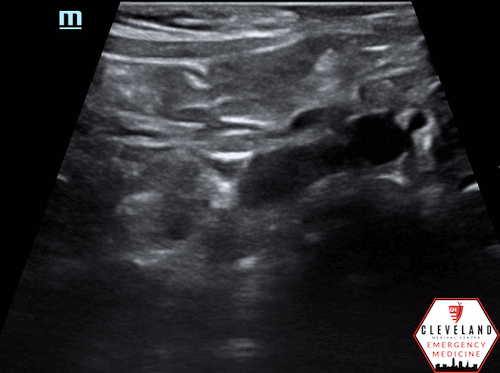
Proximal aorta viewed in transverse orientation
Mid-lower aorta
Color doppler indicating bidirectional flow
Enlarged aorta with intramural thrombus (below level of renal arteries)
Continues to increase in size when sliding probe slightly more caudally
Anterior-posterior diameter measurement
Longitudinal axis view
POCUS Findings:
An abdominal aortic aneurysm, measuring approximately 5cm in diameter, is visualized in the infrarenal region with an intramural thrombus.
Abdominal Aortic Aneurysm
Overview/Epidemiology
Abdominal aortic aneurysm (AAA) is a potentially life-threatening condition characterized by dilation of the aorta beyond its normal limits. The size of the aorta is influenced by level of the aorta, age, sex, and body size of the patient, resulting in slight variations in baseline diameter (1).
For example, a population study by Wanhainen et al. found the following average diameters:
Above the celiac artery: Males: 3.0 cm, females: 2.7 cm
Above the renal arteries: Males: 2.8 cm, females: 2.7 cm
Just below the renal arteries: Males: 2.4 cm, females: 2.2 cm
At aortic bifurcation: Males: 2.3 cm, females: 2.0 cm (2)
Despite this variability, a AAA is generally considered present when the anteroposterior (AP) diameter of the aorta exceeds 3.0 cm. Infrarenal aneurysms are most common (1).
Risk factors for AAA include older age, male gender, tobacco use, family history of aneurysm, and cardiovascular disease are associated with increased risk. The risk of rupture is higher once the aneurysm is greater than 5.5 cm or is increasing in size, if the patient has a history of tobacco use or hypertension, is of the female sex, or is symptomatic (1,3).
Clinical Presentation
The clinical presentation is widely variable. Symptoms, if present, often include abdominal, back, flank pain. However, the majority of patients are asymptomatic at the time of diagnosis, as most AAAs are found incidentally on imaging obtained for other indications or with routine screening (1).
Physical exam findings may include a pulsatile mass. While palpation alone cannot reliably diagnose or rule out AAA, the sensitivity of palpation increases with size of the AAA. In a systemic review of 15 studies involving patients not previously known to have an AAA, the sensitivity of abdominal palpation was 29% for AAA 3.0 to 3.9 cm in diameter, 50% for AAA 4.0 to 4.9 cm, and 76% for AAA 5.0 cm or greater (4). Additionally, patients may present with limb ischemia if associated with thrombus/embolus.
If AAA rupture has occurred, common symptoms include sudden onset back or abdominal pain, associated tenderness, and syncope. Flank ecchymosis (Grey Turner sign) may develop, and hemodynamic instability and hemorrhagic shock may often ensues (1).
Diagnostic Imaging
Ultrasound is the recommended screening modality based on the United States Preventive Services Task Force Recommendation Statement (5), while CT angiography (CTA) is considered a gold standard for diagnosis and perioperative planning (1). When AAA is suspected, the initial diagnostic imaging modality should be dependent on patient stability. If hemodynamically stable, CT is generally the test of choice as it has greater accuracy and is also more comprehensive in evaluating for other causes. However, if the patient is unstable, a focused ultrasound study at the bedside is preferred.
A study by Costantino et al. found point-of-care ultrasound (POCUS) performed by emergency medicine (EM) residents to have a sensitivity of 94% and specificity of 100%. In addition, they found a 4.4 mm difference in ultrasound measurements compared to CT measurements by radiologists (6). Another study by Foo et al. that also compared CT and ultrasound in patients with AAA>5.0 cm found that there was a significantly greater difference in ultrasound measurements between imaging modalities for patients with AAAs 5.0-5.4cm compared to those >5.5 cm. This suggests that the diagnostic utility of ultrasound may be more limited for smaller aneurysms (7). A more recent systematic review, including seven studies and over 650 patients, looking at the diagnostic accuracy of POCUS for AAA showed a pooled sensitivity and specificity of 99 and 98%, respectively (8). Collectively, these studies support that ultrasound is a valuable diagnostic tool for emergency physicians, including residents, to quickly evaluating for AAA size.
Management
Surgical repair is recommended for patients have symptoms attributable to their AAA, regardless of size. For asymptomatic AAA < 5.5 cm, conservative management is indicated. This includes smoking cessation, medication/comorbidity optimization, and surveillance imaging using ultrasound or CT every 6 months for aneurysms 5.0-5.4 cm, every 12 months if 4.0-4.9 cm, and less frequently for smaller aneurysms. Elective repair is most effective for preventing rupture but is not indicated until the risk of rupture outweighs risks of surgery. Once AAA exceeds 5.5 cm, repair is generally indicated due to increased risk of rupture but taking other risk factors into account. Earlier elective repair may be appropriate in certain circumstances including rapidly expanding AAA (>0.5 cm in six months or >1 cm per year), female sex, saccular aneurysm, coexistent aneurysm or peripheral artery disease (1, 9).
Point-of-Care Ultrasound of the Aorta
Key Clinical Question: Is there an aortic aneurysm?
We’ll focus on the abdominal aorta here.
Technique
Use a low frequency transducer (curvilinear probe is ideal but phased array also works) and use an abdominal preset on your machine
Place the patient in a supine position
Place the transducer just below the xiphoid process in a transverse orientation with the indicator pointing toward the patient’s right
Identify the vertebral body, which appears as a hyperechoic arch with dense shadowing posterior to it. Just anterior to this is the aorta on the patient’s left and the inferior vena cava (IVC) on the patient’s right.
Evaluate the abdominal aorta in a transverse plane from the level of the celiac trunk down to the bifurcation. Ideally, you can visualize the entirety of the aorta but, at mimimum, should obtain views of the following segments:
Proximal aorta: at level of the celiac trunk or liver tip
Mid aorta: below the SMA branch point
Distal aorta: proximal to the bifurcation
Bifurcation to the iliac arteries
Measure the anterior-posterior (AP) diameter of the aorta from outer wall to outer wall in the transverse view. A normal aorta is less than 3 cm. Normal measurements of the iliac arteries should each be less than 1.5 cm.
It is also important to include a longitudinal view of the aorta. This can be obtained by rotating the transducer 90 degrees so the probe marker points toward the patient’s head. This helps characterize the type of aneurysm (fusiform vs saccular) and potentially visualize areas that might have been missed in the transverse view (10-11).
Figure 1. Probe placement (10)
Figure 1. Transverse view of the proximal aorta at the level of the celiac trunk. Image obtained from https://www.acep.org/sonoguide/basic/aorta.
Figure 2. Longitudinal view at the level of the celiac trunk and superior mesenteric artery. Image obtained from https://www.acep.org/sonoguide/basic/aorta.
Figure 3. Transverse view of the aorta at the level of the superior mesenteric artery. Image obtained from: https://www.pocus101.com/aorta-ultrasound-made-easy-step-by-step-guide/
Distal aorta to the bifurcation into iliac arteries
Overcoming Bowel Gas: Some Helpful Tips
Bowel gas is often present and can significantly limit visualization of the aorta. Here’s a few ways to try to overcome this:
Apply gentle, downward pressure with the transducer for up to 1-2 minutes to help displace bowel gas. This is referred to as graded compression.
Consider repositioning patient in the right decubitus position to help move bowel gas away from the aorta.
If having difficulty visualizing the distal aorta or bifurcation, you can move the probe below the umbilicus and fan the probe so that it’s directed cephalad.
Can also try to view the aorta via an oblique angle with the probe placed lateral to the aorta but pointing toward midline (10-11)
Key Pathology, Pearls, Pitfalls
AAA is diagnosed when the diameter exceeds 3.0 cm. For iliac arteries, an aneurysm is when their diameter is > 1.5cm.
An intramural thrombus is often present and typically has an echogenic appearance. As a result, the inner rim may be mistaken for the aortic wall, which can lead to a falsely normal measurement if only the patent lumen is measured. This is why measuring outer wall to outer wall is important.
Some aortas have a tortuous course, so it is important to measure AP diameter to minimize risk of getting an oblique measurement
Color doppler may show turbulent or bidirectional flow. When a thrombus is present, decreased or absent flow may be seen (10-11).
Signs of rupture are often not easily seen on POCUS, as it is limited in evaluating the retroperitoneum. Some potential findings may include AAA deformation, hemoperitoneum, para-aortic hemorrhage, retroperitoneal hematoma (12).
——
Case Conclusion:
CT showed similar size and extent of her AAA compared to prior studies. Given her symptoms, vascular surgery was consulted to evaluate the patient in the emergency department. Surgery opted to hold off on emergent intervention but recommended strict blood pressure (BP) and heart rate (HR) control and planned to follow the patient. The patient was started on esmolol and nicardipine with systolic BP goal < 120 mmHg and HR < 70 bpm. She was continued on aspirin 81 mg, Plavix 75 mg, and Rosuvastatin 40 mg daily. Vascular surgery planned to perform endovascular aortic repair (EVAR). However, the patient developed a urinary tract infection, and due to risk of graft infection, outpatient repair was scheduled once her infection improved.
AUTHORED BY: ANNIE THAI, MD, PGY1
FACULTY CO-AUTHOR/EDITOR: LAUREN MCCAFFERTY, MD
References
Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A Collaborative Report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines. Circulation. 2006; 113(11):e463-654
Wanhainen A, Themudo R, Ahlström H, Lind L, Johansson L. Thoracic and abdominal aortic dimension in 70-year-old men and women--a population-based whole-body magnetic resonance imaging (MRI) study. J Vasc Surg. 2008; 47(3):504-12.
Kent KC , Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010; 52 (3): 539-548.
Lederle FA, Simel DL. The rational clinical examination. Does this patient have abdominal aortic aneurysm? JAMA. 1999;281(1):77-82).
US Preventive Services Task Force. Screening for Abdominal Aortic Aneurysm: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322(22):2211–2218.
Costantino TG, Bruno EC, Handly N, Dean AJ. Accuracy of emergency medicine ultrasound in the evaluation of abdominal aortic aneurysm. J Emerg Med. 2005;29(4):455-60.
Foo FJ, Hammond CJ, Goldstone AR, et al. Agreement between Computed Tomography and Ultrasound on Abdominal Aortic Aneurysm and Implications on Clinical Decisions. Eur J Vasc Endovasc Surg. 2011; 42, 608-614.
Rubano E, Mehta N, Caputo W, Paladino L, Sinert R. Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013;20(2):128-38
Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(1):2-77.e2
Noble V, Nelson B. Manual of Emergency Medicine and Critical Care Ultrasound, 2nd ed. Cambridge: Cambridge UP, 2011.
Risler Z, Dean AJ, Ku BS. Abdominal aortic aneurysm (AAA). American College of Emergency Physicians Sonoguide. August 2020. Accessed January 2024. https://www.acep.org/sonoguide/basic/aorta
Catalano O, Siani A. Ruptured abdominal aortic aneurysm. Categorization of sonographic findings and report of 3 new signs. J Ultrasound Med. 2005;24(8):1077–1083