Intern Ultrasound of the Month: “Boy You Got That Heartbeat Runnin’ Away”: A Case of Ventricular Tachycardia & POCUS in Cardiac Arrest
The Case
65-year-old male with a history of heart failure and unspecified arrhythmia presented from home by EMS due to palpitations. While en route, EMS placed him on the monitor and found him to be in ventricular tachycardia (VT) with a pulse. He was hemodynamically stable and otherwise mentating normally, so he received 150 mg of Amiodarone in the field. On arrival, he was transferred to our monitors, and vitals were obtained. He appeared to be in atrial fibrillation with a heart rate ranging from 90-110, normotensive.
The patient reported that over the past few days he felt episodes of his heart “racing,” which improved with rest. To the patient’s fortune, his wife chose to stay home and called 911 when he became diaphoretic.
His initial EKG showed atrial fibrillation with rate of 90, normal intervals, and no ischemic changes. A cardiac point-of-care ultrasound (POCUS) was performed.
atrial fibrillation
episodes of nonsustained VT (NSVT)
VT
POCUS findings:
In the parasternal long axis image on the left (when the patient had rate-controlled atrial fibrillation), there is decreased LV function without RV dilation or pericardial effusion. The middle and right images capture intermittent episodes of tachycardia (nonsustained VT on the monitor). Note the difference in organized contractility.
Case continued:
The patient was started on an amiodarone drip but continued to have subsequent episodes of NSVT and remained hemodynamically stable. His labs were notable for hypokalemia and hypomagnesemia, which were repleted. Cardiology was consulted and recommended a lidocaine drip, which was initiated, and he was admitted to the cardiac intensive care unit. He went into sustained VT (hemodynamically stable) and ultimately underwent ICD placement.
Ventricular Tachycardia (Overview)
Definition/Classification
Ventricular tachycardia (VT) is a wide complex tachyarrhythmia (QRS > 120 ms) with a heart rate > 100 beats per minute.
Duration is classified as sustained vs nonsustained:
Sustained VT is defined as the wide complex rhythm lasting > 30 seconds or if hemodynamic instability occurs in less than 30 seconds.
Nonsustained VT is defined as >3 beats of ventricular origin at rate >100 bpm lasting under 30 seconds in duration [1].
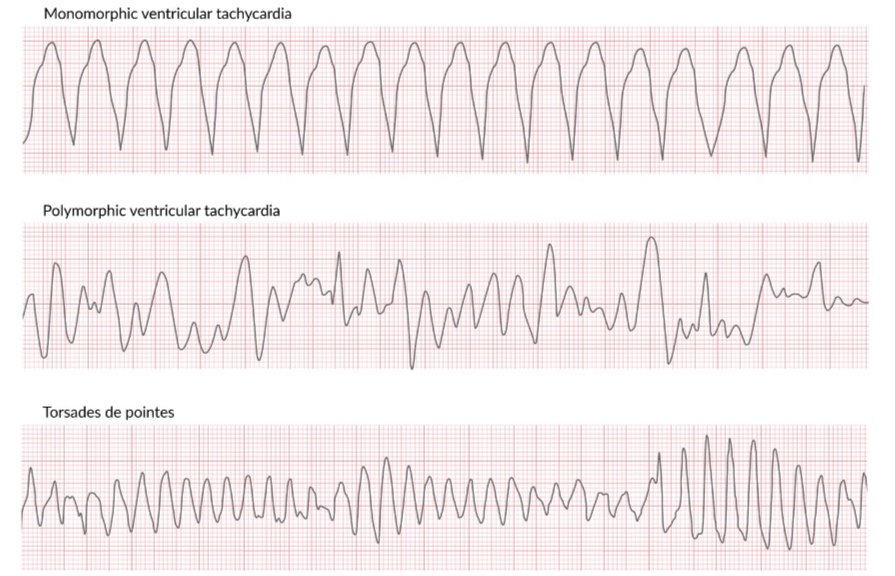
Further classification is based on QRS morphology:
Monomorphic VT shows a stable QRS from beat to beat.
Polymorphic VT has changing or variable QRS morphology from beat to beat. Torsade’s de pointes is a common example of polymorphic VT associated with long QT intervals that appears as waxing and waning amplitudes on EKG [1].
A final form of VT is bidirectional in nature, with beat-to-beat alteration. This is commonly associated with digitalis toxicity or catecholaminergic polymorphic VT [1].
Figure 2. Bidirectional VT from Digoxin toxicity
Source: https://litfl.com/bidirectional-ventricular-tachycardia-bvt-ecg-library/
Epidemiology
VT is most commonly caused by ischemic heart disease. Other causes include, but are not limited to, congenital heart disease, channelopathies, and electrolyte imbalances [1]. Most cases of sudden cardiac death can be attributed to VT and ventricular fibrillation (VF) with roughly 300,000 deaths annually in the United States, accounting for approximately half of deaths related to cardiac causes [2-3]. Risk factors for ventricular tachycardia are previous myocardial infarction, chronic obstructive pulmonary disease, ST-segment changes at presentation, and hypertension [4]. Even more concerning is VT occurring after 48 hours of hospital presentation as it is associated with increased risk of death compared to VT occurring within the first 48 hours of presentation [5].
Clinical presentation
A strong history and physical exam can help differentiate between VT and supraventricular tachycardia. Patients presenting with a past medical history of myocardial infarction, recently diagnosed angina, or heart failure have a positive predictive value of greater than 9% for VT [4]. Clinical signs and symptoms are highly variable and may include palpitations, altered mental status, diaphoresis, hypotension, pallor, variable-intensity S1, and cannon A waves in the jugular venous waveform (due to atrial contraction against a closed tricuspid valve). EKG findings are the most diagnostic in a patient’s presentation [1,4].
Management
Management is highly dependent on multiple factors including VT classification, hemodynamic stability, and underlying comorbidities and can range from close observation to pharmacologic therapy alone or in combination with cardioversion or defibrillation. For patients who survive, an implantable cardiac defibrillator or ablation is often indicated [1].
While this patient fortunately remained stable, VT can be a fatal arrhythmia so we’ll focus the rest of the discussion below on using POCUS in cardiac arrest as this is more commonly discussed in the literature.
POCUS in Cardiac Arrest
Utility
The use of POCUS in cardiac arrest and resuscitation of critically ill patients is recognized as a core skill by major governing organizations. It can be incredibly useful in differentiating organized cardiac rhythm from ventricular fibrillation, asystole, and pulseless electrical activity (PEA) and in addition to identifying reversible causes of arrest [6]. Gaspari et al. demonstrated that sonographic identification and treatment of a reversible cause of cardiac arrest increases survival. In patients with nonshockable rhythms, POCUS has significant utility in predicting worse survival outcomes in patients with cardiac standstill, i.e. no cardiac activity witnessed with ultrasound [7]. Along with its diagnostic utility, POCUS also has a vital role in procedural guidance prior to and after return of spontaneous circulation (ROSC) [6].
An additional benefit of POCUS in cardiac arrest is determining efficacy of chest compressions by providing direct, real-time visualization of cardiac chambers and surrounding structures. If the left ventricle is not adequately compressed with CPR but other structures are, this can facilitate adjustment of compressor hand placement to a more appropriate position [8].
Figure 3. CASA Protocol [10]
A Protocolized Approach
Despite its benefits, the use of POCUS in cardiac arrest as been associated with prolonged pulse checks [9]. In 2018, a novel protocol known as the Cardiac Arrest Sonographic Assessment (CASA) was developed to efficiently incorporate POCUS in cardiac arrest while maintaining high-quality cardiopulmonary resuscitation (CPR); see Figure 3. [10]
The CASA exam implements a three-step approach to rapidly evaluate for:
Cardiac tamponade
Right heart strain secondary to pulmonary embolism
Cardiac activity*
The “optimal” view will depend on the patient’s anatomy and is the one(s) that provides the focused answers you need. Only one view should be performed per pulse check and should be accomplished in under ten seconds [10].
By utilizing this stepwise approach, Clattenburg et al. showed significant reduction in pulse check interruptions by over three seconds. They also showed that having the probe on the chest in the desired location prior to pausing compressions can reduce pulse check duration while also improving image acquisition [11]. Other tips to minimize interruptions in compressions include: recording cardiac activity during the pulse check but waiting to review until after compressions have resumed (see Figure 4) and having the correct probe, machine settings (i.e. presets, depth, gain) and a towel to wipe off the gel ready ahead of time [12].
Adjuncts to the key steps of the CASA exam, which can be performed with ongoing compressions, include a pneumothorax evaluation and focused assessment with sonography in trauma (FAST) exam to help identify non-cardiac causes of arrest [10].
Protocols for repeating POCUS during cardiac arrest have not been well-studied but can be helpful if after an intervention to determine response or after a change in status.
*When evaluating for cardiac activity and none is visualized, m-mode can help confirm the presence or absence of cardiac movement, see Figure 4 [12].
Figure 4. Model to minimize delays when using POCUS in cardiac arrest [13]
Figure 5. Using M-mode to evaluate for cardiac movement [12]
Take Home Points
Ventricular tachycardia is a potentially fatal arrhythmia most commonly caused by ischemic heart disease.
POCUS is a safe, reproducible, and cost-effective tool that can quickly provide useful diagnostic information to guide management of critically ill patients, including those in cardiac arrest.
A protocolized approach, such as the CASA exam, can help optimize integration of POCUS in the resuscitative process maintaining high quality CPR.
POCUS can rapidly and effectively evaluate for cardiac tamponade, right heart strain (and other reversible causes), and cardiac activity.
Goal is to avoid interrupting or delaying resuscitation. Tips to minimize pulse check delays include: having the probe in place beforehand, recording a single window during pulse check, and reviewing images after CPR has resumed.
POST BY: DR. POLLY WILTZ (R1)
FACULTY CO-AUTHOR/EDITOR: LAUREN MCCAFFERTY, MD
Resources
Foth C, Gangwani MK, Alvey H. Ventricular Tachycardia. [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan.
Tang PT, Shenasa M, Boyle NG. Ventricular Arrhythmias and Sudden Cardiac Death. Card Electrophysiol Clin. 2017;9(4):693-708.
McNally B, Robb R, Mehta M, et al. Out-of-hospital cardiac arrest surveillance --- Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005--December 31, 2010. MMWR Surveill Summ. 2011;60(8):1-19.
Pellegrini CN, Scheinman MM. Clinical management of ventricular tachycardia. Curr Probl Cardiol. 2010;35(9):453-504.
Volpi A, Cavalli A, Franzosi MG, et al. One-year prognosis of primary ventricular fibrillation complicating acute myocardial infarction. The GISSI (Gruppo Italiano per lo Studio della Streptochinasi nell'Infarto miocardico) investigators. Am J Cardiol. 1989;63(17):1174-8.
Labovitz A, Noble V, Bierig M et al. Focused cardiac ultrasound in the emergent setting: a Consensus Statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010; 23:1225–1230.
Gaspari R, Weekes A, Adhikari S, et al. Emergency department point-of-care ultrasound in out-of-hospital and in-ED cardiac arrest. Resuscitation. 2016; 109:33–39.
Ávila-Reyes, D., Acevedo-Cardona, A.O., Gómez-González, J.F. et al. Point-of-care ultrasound in cardiorespiratory arrest (POCUS-CA): narrative review article. Ultrasound J. 2021; 13, 46.
Clattenburg EJ, Wroe P, Brown S, et al. Point-of-care ultrasound use in patients with cardiac arrest is associated with prolonged cardiopulmonary resuscitation pauses: A prospective cohort study. Resuscitation. 2017; 122: 65-68.
Gardner K, Clattenburg E, Wroe P, et al. The Cardiac Arrest Sonographic Assessment (CASA) exam—a standardized approach to the use of ultrasound in PEA. Am J Emerg Med. 2018; 36:729–731.
Clattenburg E, Wroe P, Gardner K, et al. Implementation of the Cardiac Arrest Sonographic Assessment (CASA) protocol for patients with cardiac arrest is associated with shorter CPR pulse checks. Resuscitation. 2018; 131:69–73.
Hussein, L., Rehman, M.A., Sajid, R. et al. Bedside ultrasound in cardiac standstill: a clinical review. Ultrasound J. 2019; 11, 35.
Neasi E, Kuttab HI. Use of ultrasound in cardiac arrest. ACEP Emergency Ultrasound Newsletter. 2022 Jan 31. <https://www.acep.org/emultrasound/newsroom/january-2022/use-of-ultrasound-in-cardiac-arrest2/>