Thoracic Ultrasound & Complex Pleural Effusions
The Case
68-year-old male with history of metastatic lung cancer for which he was receiving chemotherapy presented to the emergency department for 3 days of shortness of breath and chest pain/tightness. The pain was non-radiating and persisted without alleviation. He also reported a cough productive of white/yellow sputum. These symptoms were similar to his prior hospitalization for a large pleural effusion with lung collapse. He denied any other symptoms including fevers, chills, leg swelling, nausea, vomiting, diarrhea, abdominal pain, numbness, weakness, headache, URI symptoms.
In the ED, his vitals were stable aside from mild hypoxia, for which he required 2L nasal cannula. He was well-appearing and resting comfortably in bed. His breathing was not labored but he had absent breath sounds throughout his left lung. The right lung was clear to auscultation. He appeared euvolemic. The remainder of his exam was unremarkable.
Point-of-care ultrasound (POCUS) of his lungs and heart were performed and showed the following:
POCUS Findings
Lung ultrasound revealed consolidation throughout the majority of the left lung along with a complex-appearing/loculated pleural effusion in the lung base. The right lung was unremarkable.
Cardiac ultrasound was significant for a circumferential pericardial effusion without sonographic signs of tamponade. Left ventricular function was grossly normal and there were no signs of right heart strain. A distended, minimally collapsable IVC was also visualized.
(You can see the abnormal lung findings in the parasternal view)
Case continued
Chest x-ray showed white out of his left lung. CT PE study confirmed a loculated pleural effusion and progression of his extensive tumor burden resulting in occlusion of the left main bronchus and blood supply. He was admitted to the oncology service for further management. Because of his significant tumor burden and lack of perfusion to the associated lung, he was managed conservatively as it was felt that symptomatic relief would not be achieved by thoracentesis or other intervention.
Pleural Effusions, a Review
What is it?
Pleural effusions are collections of fluid between the parietal and visceral pleura of the lung. They are commonly secondary to underlying medical conditions, such as congestive heart failure, cancer, and pneumonia, though the differential remains broad [1-2].
Classification
Classification of pleural effusions can be accomplished through content (transudative vs exudative) and appearance (simple vs complex).
Effusion content is classically defined by Light’s Criteria
Sample/serum protein >0.5 or LDH ratio >0.6, or LDH >2/3 the upper limit of normal defines a exudative effusion; if it does not meet any of these criteria, it is classified as transudative [3]
Newer systems for classification have gone on to use pro-BNP levels and ratios, particularly for heart failure, as the early use of diuretics can lead to misclassification in 25% of patients [3,4]
There are several subcategories of exudative effusions (a few described below). In addition to Light’s Criteria, these can often be differentiated through additional characteristics and diagnostic testing.
Malignant effusion is associated with malignancy, most commonly lung cancer, breast cancer, or lymphoma, and contains neoplastic cells through contiguous, hematogenous, or lymphatic spread.
Empyema is a pus-containing pocket that contains inflammatory cells and/or bacteria that is secondary to infection, occasionally the primary infection, or rarely autoimmune disorders
Chylothorax is a collection of lymphatic drainage, most commonly secondary to surgical exploration of the thorax and characterized by white, milky appearance with elevated lipids
Hemothorax is a collection of blood or clot that is due to vascular injury, most commonly trauma [2]
Simple effusions are contiguous, homogeneous collections of fluid within the pleural space, while complex (often referred to as loculated) effusions tend to be septated and contain multiple compartments of fluid in between layers of fibrosis or congealed matter.
These classifications are vital for diagnostic and therapeutic purposes.
Simple effusions may be more easily drained with bedside thoracentesis, while more complex effusions frequently warrant more advanced interventions, such as video assisted thoracoscopy (VATS) or interventional radiology placed thoracostomies [2].
Epidemiology
Pleural effusions develop in 1.5 million patients per year [1]. Of these, 125,000 are malignant pleural effusions, which carries an 11.6% in-hospital mortality [5]. This can be one of the initial findings in 15% of new or recurrent diagnosis of malignancy [6].
Clinical Presentation
Clinical presentation of pleural effusion depends on the quantity of fluid as well as the underlying cause. Many patients are asymptomatic, especially if the effusion is small. Symptomatic patients commonly present with pleuritic chest pain and dyspnea. If the effusion is massive, respiratory compromise may develop. Patients may also have a wide array of additional symptoms (such as fever, weight changes, orthopnea, leg swelling, nausea) depending on the underlying etiology.
A history of malignancy, recent surgery or trauma, infection, changes in medications, and underlying health conditions some of the key historical features that may help identify the cause of the effusion and suggest potential concurrent signs and symptoms.
On physical exam, patients typically have decreased breath sounds with dullness to percussion over the affected lung fields. Additional findings may also be present depending on associated or underlying conditions [2].
Diagnostics
In addition to obtaining a thorough history and exam, diagnosis of a pleural effusion includes imaging as well as sampling of the fluid to determine underlying cause.
Common imaging modalities include chest x-ray, CT chest, ultrasound
Chest x-ray — findings include blunting of the costophrenic angle if small, basilar opacity or apparent elevation of the hemidiaphragm, and up to complete obscuration of the lung field depending on the quantity of fluid present [2].
It is important to note that findings can be ambiguous and may be indistinguishable from pneumonia or other processes. Additionally, x-rays are generally unable to differentiate the type of effusion.
CT chest (with contrast) — CT can further characterize the effusion, suggest etiology, and differentiate from solid structures, such as lymph nodes, nodules/masses, or consolidation [2]. CT is frequently indicated if clinical suspicion or surgical planning is necessary [7].
Ultrasound — even small pleural effusions can be detected with good accuracy using ultrasonography. Similar to CT, ultrasound can help differentiate fluid vs solid lesions and can suggest transudative vs exudative etiology [2, 8-9]. This assessment is discussed in more detail below.
Diagnostic evaluation of a pleural fluid is recommended in all new pleural effusions without a known etiology or if there is high suspicion for malignancy based on history and physical
Pleural fluid testing should include LDH, protein, cell count, gram stain and culture, triglycerides, and pH [10]
Additional serum testing for pro-BNP, CMP, LDH and CBC, as well as calculation of Light’s Criteria, are generally recommended [10,11]
POCUS for Pleural Effusion
Why POCUS?
POCUS allows for rapid evaluation and further characterization of a pleural effusion among other etiologies (with greater sensitivity compared to chest x-ray), which can help expedite workup and appropriate management [8-9].
Additionally, if therapeutic or diagnostic thoracentesis is required, use of ultrasound-guidance can reduce the rate of pneumothorax and other complications while improving success rates [12]. A meta-analysis by Gordon et al demonstrated an odds ratio of 0.3 in reducing pneumothorax rates [13].
General Lung Ultrasound Overview
A number of proposed techniques exist for sonographic evaluation of the thorax with some being more extensive, though time-intensive, than others [14].
A complete lung assessment generally involves a systematic approach to assessing the superior and inferior aspects of the anterior, lateral and posterior lung fields - see Figure 1. [15]
When evaluating for pleural effusions, particular attention should be given to the lung bases, as this is where fluid typically collects first.
Figure 1. POCUS Zones for Lung Evaluation, an example protocol [15]
Different probes can be used depending on the area of interest. When assessing for deeper structures, such as pleural effusions, consolidations, or B lines, a lower frequency (curvilinear or phased array) transducer is best as these allow for adequate depth visualization. When focusing more on the pleural line, a high frequency linear probe may be used [15-17].
Place the probe over the chest with the probe marker pointing toward the patient’s head. This allows you to more clearly identify the pleural line between ribs. You can then rotate the probe to a transverse orientation to better fit between ribs to improve visualization of that particular area [16]
Regardless of location or orientation, it is best to keep the transducer perpendicular to the chest wall to optimize accuracy of findings [15-16].
Normal Findings
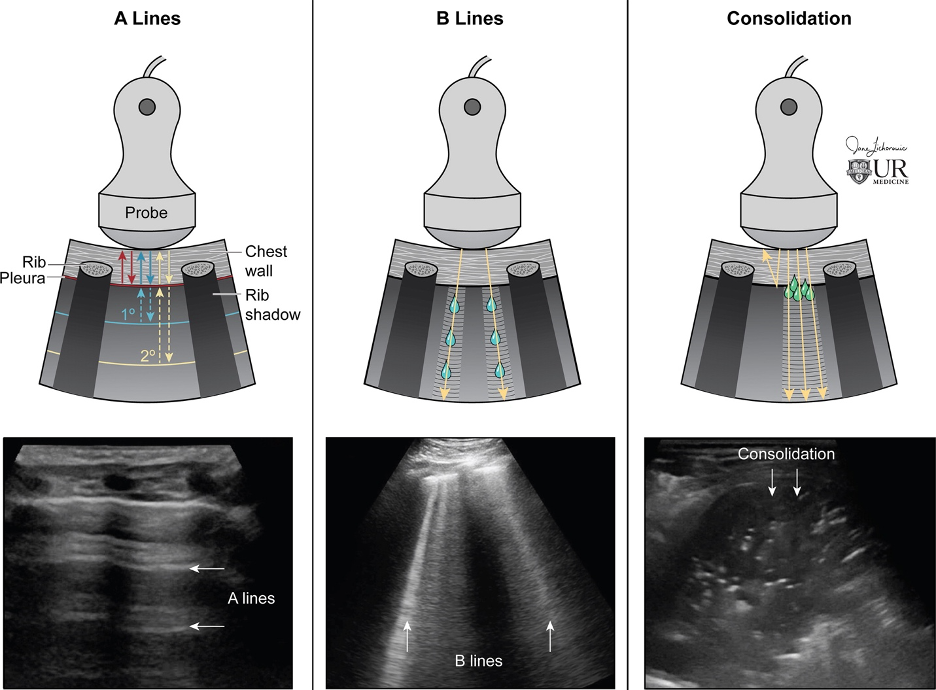
In a normal air-filled lung, the lung itself is not visualized as air scatters the sound waves, which reflect off the pleura and back to the probe. This creates A lines, which are equally spaced horizontal lines below the pleural line; while they indicate an aerated lung, they can be present in some pathologic conditions [15-16] - see Figure 2.
Figure 2. Probe Positioning with Normal and Abnormal Findings [15]
At the lung-diaphragm/liver or spleen interface, the air in a normal lung prevents visualization of the spine. As a result, the spine is only visualized below the level of the diaphragm.
Figure 3. Normal lung base. Note that the spine is not visualized above the diaphragm due to the normally aerated lung
B lines
When the intersitium fills with fluid or thickens, B lines start to appear. These are vertical hyperechoic lines emanating from the pleural surface to the bottom of the image. More than 3 per lung field are considered pathologic and the amount of B lines correlates with severity of disease.
Consolidation
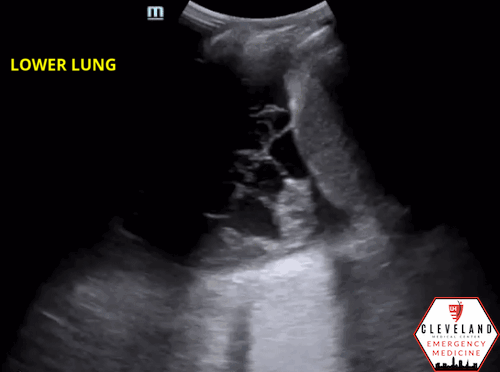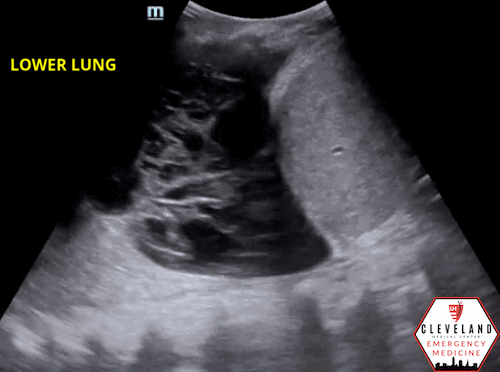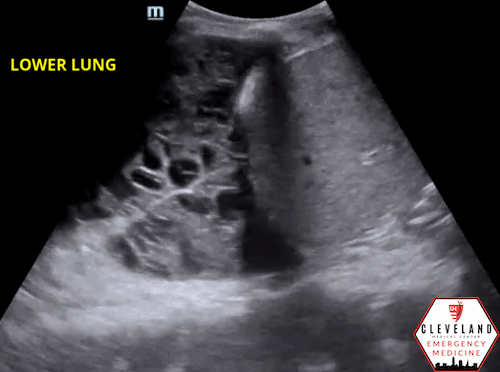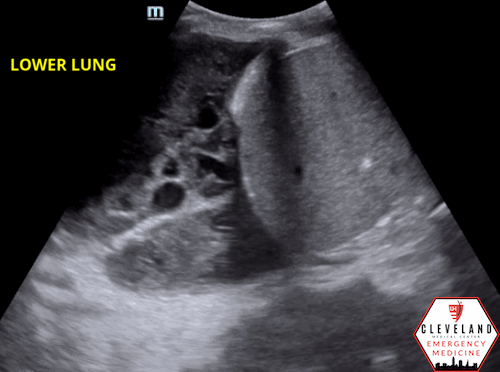
As fluid or cells fill the alveoli, areas of consolidation start to replace normal lung. Smaller consolidations may appear as focal hypoechoic areas just below the pleural line, while larger consolidations tend to develop an organ-like appearance (“hepatization”), as seen in the majority of the lung in the case above . Air that gets trapped in the fluid-filled consolidation may be visualized as bright echoes; if they move with respiration, it is a dynamic air bronchogram which is considered pathognomonic for pneumonia. Irregular borders are often seen. [15-16].
Pleural Effusion
Identification
While large effusions may be readily visualized throughout much of the lung fields, smaller effusions require more careful evaluation.
At the lung-diaphragm interface, when fluid replaces air, the vertebral bodies can now be visualized above the diaphragm. This is referred to as a spine sign - see Figure 4 [15].
A pleural effusion may also appear as anechoic fluid between the pleural line and a relatively parallel, second hyperechoic line below it. [14, 16]
Figure 4. Pleural effusion resulting in the spine sign, in which the fluid allows for visualization of the spine above the diaphragm. This fluid is anechoic and homogenous.
Figure 5. Small pleural effusion seen below pleural line, separating the parietal and visceral pleura [14]
Sonographic findings can further characterize the effusion and indicate etiology
Anechoic effusions can be transudative or exudative
Complex effusions are always exudative
Echogenicity which may demonstrate a swirling pattern — this is often seen with malignancy-associated effusions
Fibrous septations or loculations
May also see associated pleural thickening or consolidated tissue [12, 15, 17]
Figure 6. Loculated effusion (from case above)
Figure 7. Parapneumonic effusion
Useful Adjunct: Cardiac Assessment
Though cardiac POCUS does not directly assess the lungs, it can provide vital evidence for underlying etiology, such as presence or absence of decreased ejection fraction and heart failure.
Take Home Points
Pleural effusions are fairly common and are secondary to an underlying condition, most commonly heart failure, pneumonia, and cancer.
Classification of pleural effusions is determined by appearance and fluid characteristics (i.e. Light’s Criteria)
POCUS is a fast, reliable way to assess for pleural effusions (among other etiologies for dyspnea), is more sensitive than x-ray, and does not involve radiation exposure.
Ultrasound-guided thoracentesis is recommended to reduce the risks of pneumothorax and other complications.
POST BY: DR. IAN BRALLIER (R1)
FACULTY CO-AUTHOR/EDITOR: LAUREN MCCAFFERTY, MD
References
Feller-Kopman, David, and Richard Light. "Pleural disease." N Engl J Med. 2018; 378(8): 740-751.
Karkhanis VS, Joshi JM. Pleural effusion: diagnosis, treatment, and management. Open Access Emerg Med. 2012;4:31-52.
Light RW. The Light criteria: the beginning and why they are useful 40 years later. Clin Chest Med. 2013;34(1):21-26.
Porcel JM, Vives M, Cao G, Esquerda A, Rubio M, Rivas MC, Measurement of pro-brain natriuretic peptide in pleural fluid for the diagnosis of pleural effusions due to heart failure. Am J Med. 2004; 116(6):417-420
Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 National Inpatient Sample. Chest; 2017;151:84554.
Porcel JM, Gasol A, Bielsa S, Civit C, Light RW, Salud A. Clinical features and survival of lung cancer patients with pleural effusions. Respirology. 2015;20:6549.
Hallifax RJ, Haris M, Corcoran JP, et al. Role of CT in assessing pleural malignancy prior to thoracoscopy. Thorax 2015;70:192-193.
Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816.
Yang PC, Luh KT, Chang DB, Wu HD, Yu CJ, Kuo SH. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roent Genol. 1992;159:2933.
Porcel JM, Vives M, Vicente de Vera MC, Cao G, Rubio M, Rivas MC. Useful tests on pleural fluid that distinguish transudates from exudates. Ann Clin Bio Chem. 2001;38:6715.
Kolditz M, Halank M, Schiemanck CS, Schmeisser A, Höff ken G. High diagnostic accuracy of NTproBNP for cardiac origin of pleural effusions. Eur Respir J. 2006;28:14450.
Havelock T, Teoh R, Laws D, Gleeson F. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65: Suppl 2:ii61ii76.
Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339.
Lichtenstein DA. Lung ultrasound in the critically ill. Ann. Intensive Care. 2014; 4(1):1.
Marini TJ, Rubens DJ, Zhao YT, et al. Lung Ultrasound: The Essentials. Radiol Cardiothorac Imaging. 2021; 3:2.
Rambhia SH, D’Agostino CA, Noor A, et al. Thoracic Ultrasound: Technique, Applications, and Interpretation. Curr Probl Diagn Radiol. 2017; 46(4): 305-316.
Hassan M, Mercer RM, Rahman NM. Thoracic ultrasound in the modern management of pleural disease. Eur Respir Review. 2020; 29 (156): 1-11.