Intern Ultrasound of the Month: Left Ventricular Hypertrophy
The Case
25-year-old male with past medical history including Down syndrome, remote repair of aortic coarctation, hypertrophic cardiomyopathy presented for a non-productive cough and URI symptoms for the past 2 days. His mother accompanied the patient and provided additional history as the patient is nonverbal at baseline. She stated that he also had a poor appetite and intermittent chest pain, which she attributed to his cough. Otherwise she denied fever, syncope, shortness of breath, leg swelling, GI symptoms, known sick contacts. She believed he been taking his home medications.
His vitals were all within normal limits. Physical exam was unremarkable aside from an occasional cough. He appeared euvolemic and his lungs were clear.
EKG demonstrated normal sinus rhythm, normal rate, left axis deviation, and right bundle branch block that appeared unchanged from prior EKG. Significant lab findings included an elevated high sensitivity troponin of 150 ng/L and positive influenza test.
Though the patient’s presentation was attributed to a viral illness, a point-of-care ultrasound (POCUS) was performed to evaluate for cardiac pathology in the setting of elevated troponin, chest pain, and his prior history.
POCUS Findings
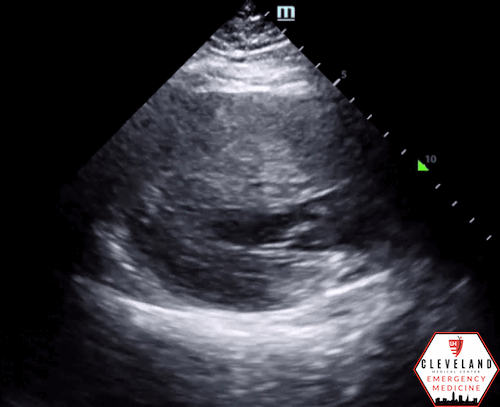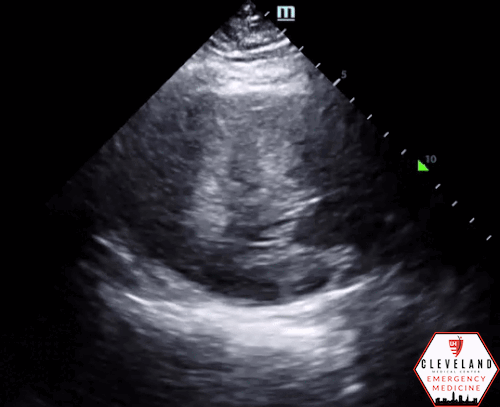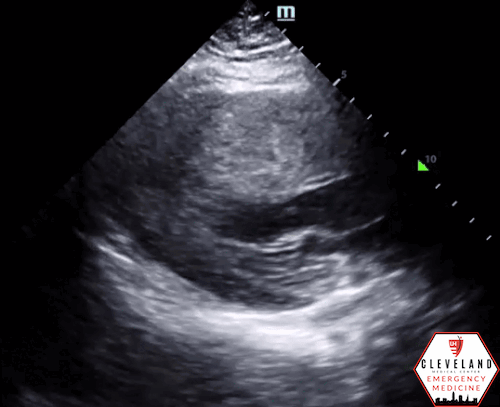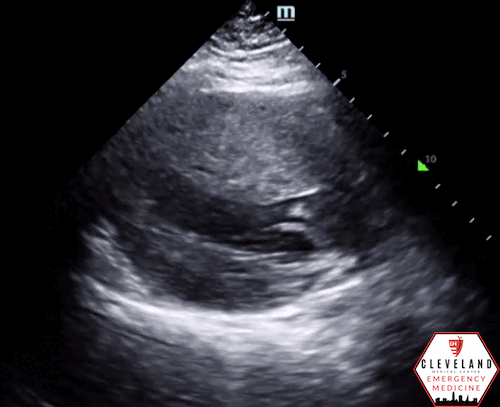
Left ventricular function is relatively normal-appearing without overt evidence of right ventricular enlargement or pericardial effusion. However, the left ventricle is significantly hypertrophic, particularly the interventricular septum (consistent with his history of hypertrophic obstructive cardiomyopathy (HOCM). There were no prior echocardiograms available for comparison.
Case Continued
Given the focused echo findings along with his elevated troponin and cardiac history, cardiology was consulted. They attributed the mildly elevated troponin to his severe left ventricular hypertrophy and associated microvascular disease rather than acute coronary syndrome. In the absence of syncope, they deferred urgent workup or ICD placement. A comprehensive transthoracic echo (TTE) was performed while the patient was in the ED and showed normal left ventricular (LV) systolic function with severe concentric left ventricular hypertrophy with an asymmetrically thickened septum and decreased LV cavity size. Additionally, he was noted to have systolic anterior motion (SAM) of the mitral valve - indicating outflow tract obstruction - along with reduced right ventricular systolic function and evidence of remote surgical repair. The patient remained stable and outpatient evaluation for ICD placement with congenital cardiology was felt to be appropriate. He was advised to continue taking his home beta blocker and calcium channel blocker in the meantime.
Left Ventricular Hypertrophy
Background
Left ventricular hypertrophy (LVH) is a heart condition that results in an increase in the mass of the LV due an increase in wall thickness and/or enlargement of the LV cavity itself. Left ventricular wall thickening occurs in response to pressure overload, while chamber dilation occurs in response to volume overload [1]. LVH is present in approximately 15-20% of the general population and is more prevalent in African Americans, the elderly, and patients with obesity or hypertension. LVH is equally common in women and men [2].
Multiple etiologies can lead to the development of LVH, the most common of which are hypertension and aortic valve disease due to the constant compensation of hemodynamic pressure and volume burden. While LVH may be a compensatory mechanism, it is still abnormal. There is development of an abnormal increase in the LV’s myocardial mass due to a chronically elevated workload on the heart muscle [3]. This pathologic hypertrophy then puts patients at risk of developing heart failure, dysrhythmias and sudden cardiac death. Other common causes of LVH include athletic heart with physiological LVH, ventricular septal defect, coarctation of the aorta, hypertrophic cardiomyopathy, renal artery stenosis and infiltrative cardiac diseases [1].
It should be noted that LVH can be further classified as concentric or eccentric. Concentric LVH is an abnormal increase in left ventricular myocardial mass due to pressure overload from arteriolar vasoconstriction which occurs in chronic hypertension or aortic stenosis. Eccentric LVH, on the other hand, is from increased filling pressure of the left ventricle or diastolic overload. This arises from valvular regurgitation and cases of dilated cardiomyopathy [1].
Clinical Presentation
LVH is difficult to diagnose based off signs and symptoms alone, as the disease process itself reflects a pathophysiologic response over time of the heart to pressure/volume overload. As such, the signs and symptoms are instead related to the underlying pathologic cause of LVH, such as hypertension and abnormal heart sounds, e.g. murmurs, from valvular pathology [4]. Patients may be able to compensate for years with no overt symptoms and nearly normal exercise reserve. Other patients may go on to develop heart failure from diastolic and/or systolic dysfunction [5].
Diagnosis
The diagnosis of LVH is largely dependent on echocardiographic measurements or non-invasive imaging techniques. Analyses from the Framingham Heart Study demonstrated that echocardiographic LVH (17.4%) is more prevalent than LVH detected by electrocardiography (2.4%) [6]. Cine-MRI and ultrafast CT have been used to detect LVH as well, however these current novel imaging studies are limited by a smaller size of study groups and less robust outcome data over time [7]. EKG findings that LVH can produce include: increased QRS duration, increased QRS voltage, left axis deviation, ST-T repolarization changes and left atrial abnormality. However, these findings though relatively specific lack sensitivity [8]. The Framingham Heart Study provided criteria for measurements of LV mass based off a healthy distribution, and thus, the basis to detection of pathological LVH based off adjustments for sex, height and body mass [6]. According to the American Society of Echocardiography and/European Association of Cardiovascular Imaging, LVH is defined as an increased left ventricular mass index (LVMI) to greater than 95 g/m in women and increased LVMI to greater than 115 g/m in men [1].
Management and Potential Complications
The management of LVH is varied and ultimately based on the underlying cause, such as hypertension, with the goal of preventing progression to heart failure and mortality. Overall, treatment is centered around lifestyle changes, medications, surgery and implantable devices. Multiple studies have shown that regression of LVH can occur with appropriate hypertension management. In turn, there is an associated reduction in potential complications such as cardiovascular death, fatal or nonfatal MI and fatal or nonfatal stroke. Additionally, changes in LVH were associated with improved parameters of diastolic function and with decreased recurrent hospitalization for heart failure [9].
POCUS for Evaluation of Left Ventricular Function:
Indications
Bedside echocardiography has invaluable benefits in the emergency department. Focused cardiac ultrasound can quickly evaluate LV function (in addition to pericardial effusion and signs of right heart strain) and help determine etiology of undifferentiated hypotension, chest pain and dyspnea, atrial fibrillation, among others [10-11]. As delineated earlier, not only is the recognition of LVH important on echocardiography, but the consequences on heart remodeling due to LVH may be even more important. Assessment of LV ejection fraction is a hallmark of assessing left ventricular function as a whole and is a valuable tool in the management of patients in the ED [10].
Evaluation and Interpretation of Left Ventricular Systolic Function
LV function is primarily expressed as an ejection fraction (EF) or the fraction of the blood present in the LV during diastole that is ejected. Three methods for LV systolic function estimation used in POCUS are visual assessment, fractional shortening (FS), and E-point septal separation (EPSS) [10-12].
1. Visual assessment:
The fastest and most practical way to estimate LVEF in the ED
LVEF is separated into 3 categories:
Normal: LVEF > 50% (hyperdynamic function: >70%)
Moderate dysfunction: LVEF 30-50%
Severe dysfunction: LVEF < 30%
Subjective visual assessment is made of the degree of contraction between systole and diastole—with the heart evaluated in at least 2 views
All visualized walls of the ventricle should move symmetrically towards the center of the heart chamber during systole and the walls should thicken as the muscle contracts
Figure 1. Normal EF
Figure 2. Mild-moderately reduced EF — note the reduced change in chamber size and wall thickening with each contraction. Mitral valve movement is somewhat decreased as well.
Figure 3. Severely reduced EF — note the significantly reduced change in chamber size and wall thickening, along with poor movement of the mitral valve
2. Fractional Shortening:
Assessment of the “squeeze” of the left ventricle
Change in anterior-posterior measurement of the LV at end of systole versus the end of diastole (expressed as a percentage)
Most reliable in parasternal short and long axis and should be assessed at level of chordae tendinae
Note that this is not the same as EF; FS is approximately half the EF in most cases
Figure 4. Fractional shortening [11]
3. E-point Septal Separation (EPSS):
The mitral valve opens and approaches the septum with every diastole
EPSS is the distance in millimeters between the anterior leaflet of the mitral valve and the interventricular septum in the parasternal long axis view during the early opening point of the mitral valve in diastole. Images are obtained in M-mode.
EPSS ≤ 6mm is seen in normal LVEF
EPSS > 7mm is seen with reduced LV function [13]
*Note that this could be falsely normal in the setting of LVH
Figure 5. EPSS, assessing mitral valve proximity to septum during diastole [11]
Figure 6. Measurement of EPSS in M-mode [11]
Pearls and Pitfalls
Methods of LV assessment are operator-dependent and it is unclear how many studies one must perform to become proficient as estimating LV function
Asymmetric wall motion abnormalities may be difficult to evaluate and LVEF is not always the best indicator of cardiac output in cases of valvular disease
Tachycardia with normal EF may mimic a hyperdynamic heart
Body habitus, hyperinflated lungs, patient positioning may impede good image quality
Scan in a systematic fashion and consider sonographic findings in the clinical context of the patient [10-11]
A Quick Word on Left Ventricular Hypertrophy
While not one of the common focused clinical questions answered with POCUS, it is important to recognize LV hypertrophy when present. LVH is defined as LV wall thickness measuring >1.5 cm [14], which can be easily assessed with POCUS. When the interventricular septum is involved, the patient is at risk of LV outflow tract obstruction.
Take Home Points
LVH is a common condition that develops in response to pressure overload and can have potentially life-threatening consequences if not recognized and addressed
Cardiac POCUS answers focused clinical questions at the bedside, and evaluating for LV function can play an important role in the workup and management of patients in the ED
Develop a systematic approach to your POCUS assessment and improve your ability to estimate LV function through practice
Pay attention to LV wall thickness and recognize hypertrophy when present (>1.5cm), especially in patients with concerning clinical findings or historical features
POST BY: DR. SHRUTI AFRICAWALA, PGY1
FACULTY CO-AUTHOR/EDITOR: LAUREN MCCAFFERTY, MD
References
Bornstein AB, Rao SS, Marwaha K. Left Ventricular Hypertrophy. [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557534/
Cuspidi C, Sala C, Negri F, Mancia G, Morganti A., Italian Society of Hypertension. Prevalence of left-ventricular hypertrophy in hypertension: an updated review of echocardiographic studies. J Hum Hypertens. 2012;26(6):343-9.
Marketou ME, Parthenakis F, Vardas PE. Pathological Left Ventricular Hypertrophy and Stem Cells: Current Evidence and New Perspectives. Stem Cells Int. 2016;2016:5720758.
Goldberger AL. Left ventricular hypertrophy: Clinical findings and ECG diagnosis. [Updated 3 November 2022]. Accessed May 2023. <https://medilib.ir/uptodate/show/2112>
Gunther S, Grossman W. Determinants of ventricular function in pressure-overload hypertrophy in man. Circulation. 1979; 59:679–688.
Levy D, Savage DD, Garrison RJ, et al. Echocardiographic criteria for left ventricular hypertrophy: the Framingham Heart Study. Am J Cardiol.1987; 59:956–960.
Lorenz CH, Walker ES, Morgan VL, et al. Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiov Magn Res.. 1999;1:7–21.
Goldberger AL, Goldberger ZD, Shvilkin A. Goldberger's Clinical Electrocardiography: A Simplified Approach, 9th ed, Elsevier/Saunders, Philadelphia 2017.
Sayin BY, Oto A. Left Ventricular Hypertrophy: Etiology-Based Therapeutic Options. Cardiol Ther. 2022; 11: 203–230.
Siadecki S, Saul T, Lewiss R, Solomon R. Bedside Ultrasound Assessment of Left Ventricular Function. ACEP Now. 3 Feb. 2015. Accessed May 2023. <www.acepnow.com/article/bedside-ultrasound-assessment-left-ventricular-function/?singlepage=1>
Woo, Michael. Point-of-Care Echocardiography for the Emergency Physician: A Primer, 1st Edition. Accessed May 2023 <emottawablog.com/wp-content/uploads/2020/07/DEMEchov3.docx-1.pdf>
Mallin M, Dawson M. Introduction to Bedside Ultrasound: Volume 2. Apple Books; 2013.
Miller T, Salerno A, Slagle D. Advanced Critical Care Ultrasound: E-Point Septal Separation to Estimate Left Ventricular Ejection Fraction. EMRA. 2021; May 2021. Accessed May 2023 <www.emra.org/emresident/article/epss>
Cunningham KS, Spears DA, Care M. Evaluation of cardiac hypertrophy in the setting of sudden cardiac death. Forensic Sci Res. 2019; 4(3): 223–240.